3/12/24
Question: Should Non-Invasive Ventilation be used routinely for preoxygenation for Emergency Intubation in the ED?
The PREOXI randomised controlled trial, has just been published in the New England Journal of Medicine evaluating the use of Non-Invasive Ventilation (NIV) for preoxygenation in patients undergoing intubation in the ICU and ED. It is an incredibly useful contribution to this topic.
For those unfamiliar with the trial details, AI has provided a brief summary.
This is then followed by my interpretation, practical tips and conclusion.
Finally, links to other reviews of the study are provided as well as a note about some concerns regarding Evidence Based Medicine (EBM) practice in the publication process.
Summary of the Trial and Its Findings
Title: Noninvasive Ventilation for Preoxygenation during Emergency Intubation
Background: The trial aimed to determine the effectiveness of noninvasive ventilation (NIV) compared to the oxygen mask for preoxygenation in critically ill adults undergoing tracheal intubation. The primary concern was to assess the incidence of hypoxaemia, defined as an oxygen saturation of less than 85% during intubation.
Methods:
- Design: Multicenter, randomized, unblinded, parallel-group trial.
- Participants: 1301 critically ill adults from 24 emergency departments and ICUs in the U.S.
- Intervention: Patients were randomized to receive either NIV or an oxygen mask for preoxygenation before intubation.
- Primary Outcome: Hypoxaemia during intubation.
- Secondary Outcomes: Lowest oxygen saturation during intubation, exploratory outcomes included hemodynamic events and safety outcomes.
Results:
- Primary Outcome: Hypoxaemia occurred in 9.1% of patients in the NIV group and 18.5% in the oxygen mask group, a difference of -9.4 percentage points (95% CI, -13.2 to -5.6; P<0.001).
- Secondary Outcome: Median lowest oxygen saturation was 99% in the NIV group and 97% in the oxygen mask group, a difference of 2 percentage points (95% CI, 1 to 3).
- Exploratory Outcomes:
- Oxygen saturation <80%: 6.2% in the NIV group vs. 13.2% in the oxygen mask group.
- Oxygen saturation <70%: 2.4% in the NIV group vs. 5.7% in the oxygen mask group.
- Cardiac arrest: 0.2% in the NIV group vs. 1.1% in the oxygen mask group.
- Safety Outcomes:
- Aspiration: 0.9% in the NIV group vs. 1.4% in the oxygen mask group.
- New opacity on chest radiography and pneumothorax were similar between groups.
Conclusion: Preoxygenation with NIV significantly reduced the incidence of hypoxaemia during intubation compared to the oxygen mask. The trial suggests that NIV is a more effective method for preoxygenation in critically ill adults undergoing tracheal intubation without increasing the risk of aspiration.
Detailed Data:
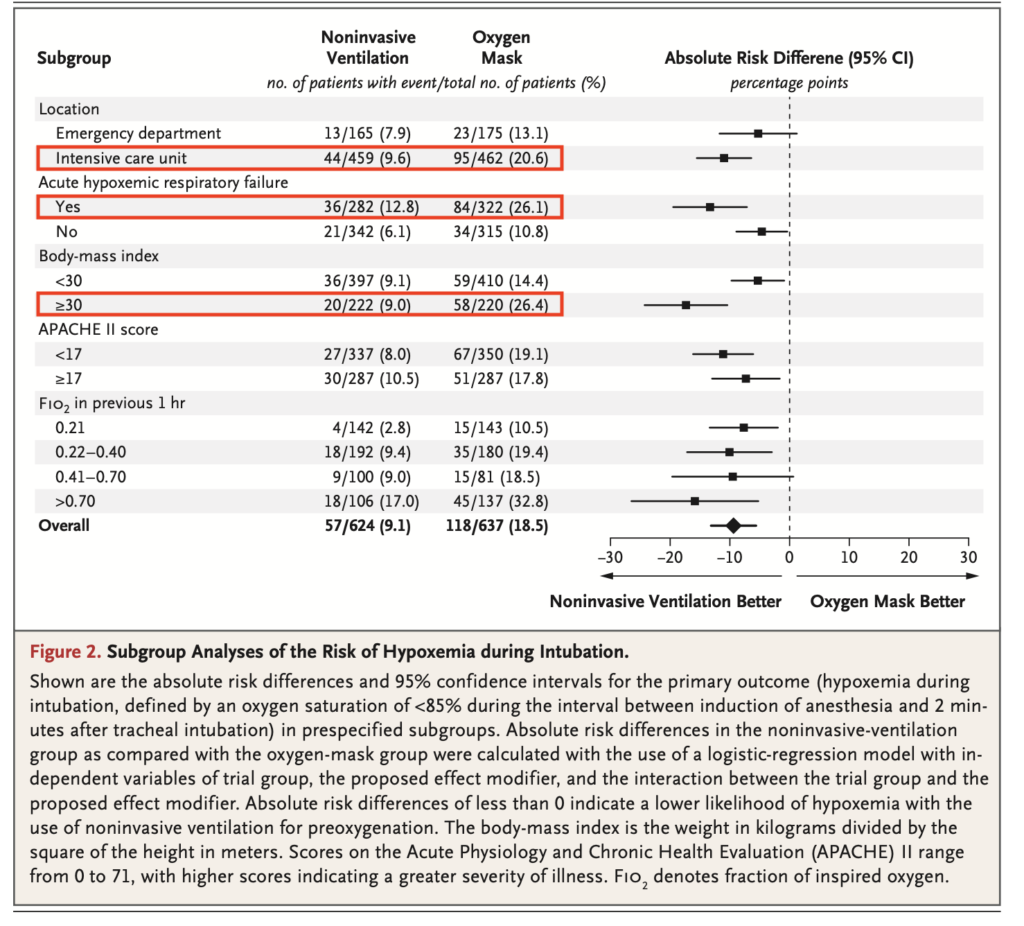
- Subgroup Analysis (Figure 2): Showed consistent benefits of NIV across various subgroups, with a notable reduction in hypoxaemia, particularly in patients with higher body mass index.
- Patient Characteristics (Table 1): Both groups were well-matched in terms of demographics and clinical characteristics.
The study provides robust evidence supporting the use of noninvasive ventilation for preoxygenation in emergency intubation of critically ill patients.
Trial Interventions
This is a fairly well conducted open-label RCT, with pragmatic study design, that clearly shows a clinically important reduction in desaturations during emergency intubation.
This is essentially just VAPOX protocol (Ventilator Assisted PreOxygenation) which we’ve described before based on an Australian case series published in 2016 (linked in that post).
The rationale for VAPOX is
- 1) Optimising pre-oxygenation through CPAP during preoxygenation and the apnoeic period post delivering sedation/paralysis.
- 2) Delivering machine controlled “safe” ventilations during the apnoeic period to minimise desaturation risk during intubation, which are preferable to adrenaline-fuelled ventilations delivered by a “hyped-up” human that may have a greater risk of insufflating the stomach and causing aspiration.
- 3) In the event of failed intubation, further machine controlled breaths can be given for re-oxygenation.
This trial provided a package of NIV care that evaluated the combined benefits of 1 and 2 (not 3).
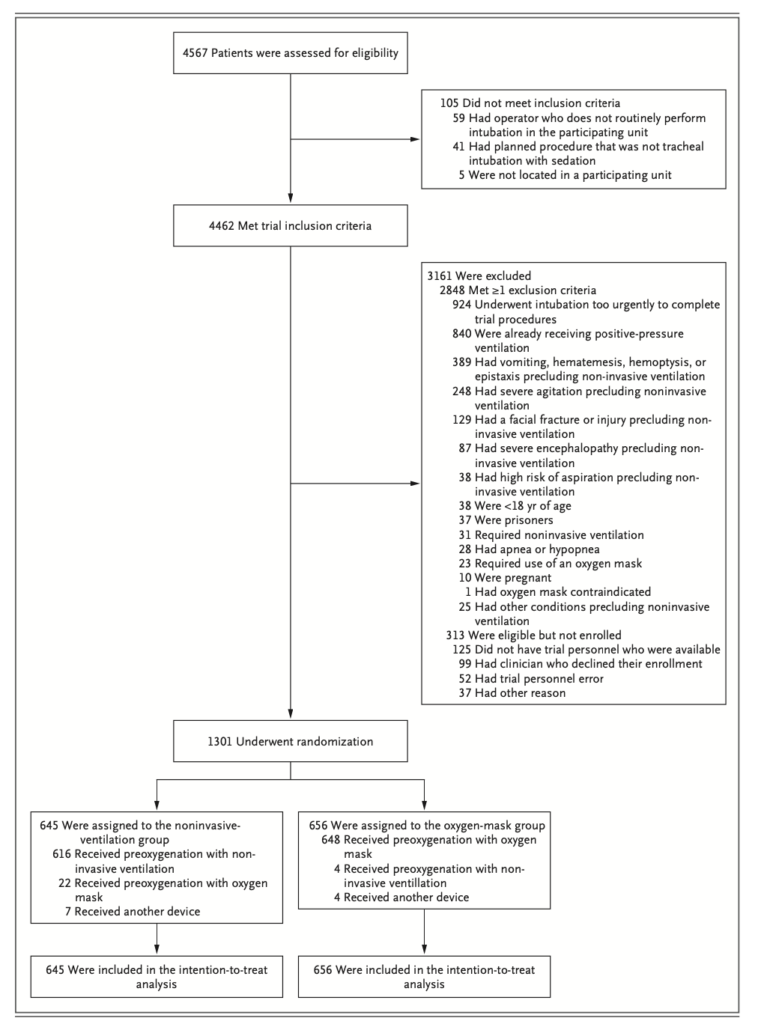
There were a lot of exclusions that are worth noting (see picture below). The most important exclusion was patients who were “already on NIV”. This seems reasonable as such patients would be considered to almost certainly benefit from NIV for preoxygenation so the purpose of this study was to look at all the other patients we intubate to determine, do they benefit too?
There are a number of peculiarities and uncertainties in the reporting of outcomes and patient groups. These observations potentially affect the generalisability of the findings. In piecing this together, I’ve included direct correspondence from the lead author Dr Kevin Gibbs and podcast interviews with key paper authors.
Some observations:
- Half the patients in the trial had hypoxaemic respiratory failure, yet none of these were on NIV prior to the intubation. Since the widespread use of NIV over the last few decades, very few patients require intubation for hypoxaemic respiratory failure because NIV works so effectively, especially in the ED (some do well in ED on NIV but later fail in ICU). This therefore seemed like a peculiar cohort, but fortunately correspondence with the lead author Kevin Gibbs provided some insights:
- In some patients High Flow Nasal Oxygenation (HFNO) may be used instead of NIV and Dr Gibbs confirmed this was the case in this cohort. Of note about 1/4 of the total patients were on HFNP which probably accounts for half of the hypoxaemic respiratory failure patients leaving the other half without either NIV or HFNO.
- Dr Gibbs advised, that where a patient with multiple problems, “say on 3L nasal prongs + sepsis + altered mental state” is intubated they may be recorded on the data sheet as having hypoxaemic respiratory failure present (due to the need for any oxygen therapy) even though this was clearly not the primary “reason for intubation”.
- So the hypoxaemic respiratory failure group might have been defined very loosely and could refer to potentially any patient requiring oxygen to achieve normal saturations, where the cause of hypoxia is not simply sub-optimal ventilation. Interestingly, if true, it potentially makes the findings more generalisable. Unfortunately, I also could not find an actual definition for this group/term in the protocol, paper or supplementary appendix and the Dr Gibbs did not reply to questions verifying if any such definition existed in the trial materials. Furthermore, while the the indication for intubation (including hypoxaemic respiratory failure) was recorded in the trial, this data was not published. Apparently this was due to a post-hoc decision to only report whether hypoxaemic respiratory failure was merely present rather than if it was the indication for intubation (see section entitled Concerns re EBM Practice for further discussion).
- This was a sick cohort with about 1/5 of the control group meeting the primary outcome of desaturation to 85% and about 1/3 of both groups dying in hospital. About 3/4 were ICU patients versus 1/4 ED patients which may explain this cohort.
- Most benefit seen in the primary outcome from NIV preoxygenation was seen in patients with obesity (BMI>30) (16% difference), hypoxaemic respiratory failure (13% difference) or were in the ICU (11% difference) compared to overall study benefit of only 9%. This aligns with what one would expect based on pathophysiology. While in general one should be cautious about interpreting sub-group analysis, this fits strongly with the prior probability of benefit in such groups. As expected the more FiO2 the patient was on before intubation, the greater the benefit from being randomised to the NIV group, but of note, even patients on room air had almost a 7% difference in the primary outcome.
The above issues leads to problems with generalisability to all patients intubated in the ED. In each of the alternative sub-groups (non-obese, ED patient, no hypoxaemic respiratory failure) the absolute benefits in the primary outcome were much smaller – only in the region of 5% compared to the overall study benefit of 9%. It is likely that where all 3 of these characteristics existed simultaneously – a non-obese ED patient without hypoxaemic respiratory failure – the benefit was likely far smaller or possibly non-existent (and was unfortunately not reported in the publication). Recall this was also only a proxy endpoint of desaturations, not actual patient harm, so I’d expect a very low probability of any actual reduction in patient harm in those patients. This may not be outweighed by the potential harms of NIV. This is critically important as non-obese ED patients without hypoxaemic respiratory failure are a large proportion of the patients we intubate.
Of interest, peri-intubation cardiac arrest was lower in the NIV group. It occurred in 1 patient (0.2%) v 7 patients in the oxygen mask group – difference 0.9% (-1.8 to -0.1) but this was also an exploratory secondary outcome and that was applied to the whole group not the less sick cohort described above. Of the 7 patients who arrested in the oxygen mask group, the indication for intubation was “hypoxaemic respiratory failure” in 4 of them [of note, this reporting on arrested patients is incidentally the only place in the published paper or supplementary appendix where indication for intubation was mentioned].
A Straw man Comparison – a sub-optimal control group
Additionally there were some potential problems with suboptimal oxygen delivery in the oxygen mask group
- Only about 1 in 5 of the oxygen mask group had simultaneous use of nasal cannula (standard or high flow) and where a BVM was used, it was not reported whether PEEP valves were used.
- It was also not reported whether the non-rebreather masks (NRM) were being run at flush rate (oxygen litres/min valve opened completely). A NRM run at the standard 15L/min only provides FiO2 in the region of 70% whereas run at flush rate provides about 90%. Apparently, according to author interviews, package education for sites did advise the use of flush rate but we don’t know if this actually occurred to any significant extent.
If nasal cannula had been used as a standard of care with all BVM’s being fitted with PEEP valves and NRM were being run at flush rate, it is quite possible that the benefit of NIV seen in this study over the oxygen mask group may have been far less, or possibly (but I suspect less likely) non-existent. So this represents the ultimate “straw man” comparison – NIV was shown to be better than a sub-optimal comparator. The counter argument is that if you notice that practice variability in your ED reflects this study with nasal cannula, PEEP valves and NRM flush rate being inconsistently applied peri-intubation, then your patients may benefit equally as the patients in this study from using NIV instead. So the flip side to the straw man critique is that the pragmatic nature of the study may mean that the comparison was actually a fair and realistic one.
Safety:
- The good news was that NIV was found to be safe in the study with no increase in aspirations. The caveat to this is there were a huge number of exclusions (see picture below) which limits the full generalisability of this safety data, including any situation where the clinician felt NIV was contraindicated.
- It has been theorised for some time that these relatively low pressure, machine controlled breaths, usually providing total inspiratory pressures no higher than 20cm H20, are unlikely to open up the lower oesophageal sphincter and cause aspiration. This trial is supportive of this.
- See the following Practical Tips section for a discussion regarding suggested ventilator pressure settings.
Exclusion Criteria Diagram from the publication
Pressure Settings (cm H20):
In the original VAPOX protocol post, initial settings suggested were CPAP/PEEP 5 + Pressure Support of 10 = IPAP (Inspiratory Positive Airway Pressure) of 15.
In this trial they also used a “minimum PEEP of 5” but only a minimum Pressure Support of 5 (minimum IPAP was only 10).
Respiratory Rate
The ventilator mode used is Bilevel ventilation/BiPAP however you need to choose a setting that provides a “back up respiratory rate” (e.g. “NIV-ST” mode in the Hamilton T1 Ventilator). This is to ensure ventilations are still provided after sedation/paralytics cause the patient to become apnoeic before intubation
The rate used in the original VAPOX protocol post was 6-8 breaths/min which should generally be enough when delivering 100% FiO2. In this study they used at least 10 breaths/min, which might not be necessary but appeared safe.
In acidotic patients the rate may need to be much higher.
Mask Use
There are 3 options for choice of mask for NIV
- 1) NIV mask strapped to patients head – as usually given with NIV delivered to alert patients
- 2) NIV mask held on a patient’s face by the intubator without strapping.
- 3) Soft plastic BVM type mask (connected to the ventilator circuit not BVM) also held on a patient’s face by the intubator without strapping.
Simultaneous jaw thrust may be required in obtunded patients to optimise airway opening and delivery of ventilation and PEEP.
The advantages of 2) or 3) are that if complications occur such as regurgitation, this can be quickly recognised and attended to. The disadvantage is that the intubator’s hands can fatigue when preoxygenating for 3-5 minutes and combined with a loss of concentration may allow mask leaks and a drop in delivered CPAP and FiO2.
The benefit of 1) is that it is simpler, potentially more effective and appeared safe in this trial. However I’d suggest this isn’t a situation of strapping on the mask and walking away. The intubator should generally be standing at the patient’s head keeping an eye out for complications. During the apnoeic period when the patient loses tone due to paralytics/sedation, jaw thrust should be strongly considered (though this wasn’t specified in the study).
Where NIV is not used, but you wish to maximise your CPAP simply using a BVM with a PEEP valve – see the Other Reviews/Resources section for how to do this.
Patient Compliance Tips
- In awake, communicative, patients, explaining the NIV procedure in advance and how it will help them can improve compliance.
- Upon starting NIV, continue to talk the patient through the process while holding the mask on their face to ensure they are comfortable with the process before strapping the mask on (where they may feel a loss of control). If they can’t tolerate the mask strapped on (Option 1), revert to option 2 or 3.
- Consider starting on lower pressures first to gain the patient’s confidence before increasing to desired target pressures.
- In combative patients lacking capacity, who are unable to comply with the required preoxygenation, either by oxygen mask or NIV, Delayed Sequence Intubation (DSI) should be considered to optimise their safety.
Based on this trial, prior experience and specialist opinion, I think the patients who would be most likely to benefit from NIV preoxygenation in ED intubation are patients from one or more of the following 4 groups:
- Obesity (BMI>30)
- Hypoxaemic respiratory failure
- Acidosis
- Anticipated Difficult Airway
Groups 1 and 2 have strong backing with evidence from this trial combined with prior probability. It may be reasonable to define group 2 quite loosely like they appeared to in the trial (sadly this remains unconfirmed) as any patient requiring oxygen to achieve normal saturations where the cause was not simply sub-optimal ventilation. Defining this group remains a primary point of conjecture in this study and its application to our patients.
Group 3 would likely benefit from increased ventilation, before and most importantly during, the apnoeic period to blow off CO2 (though this group was not specifically evaluated in this study). Note they would need a much higher back up respiratory rate than the suggested 6-10 breaths/min to take them through the apnoeic period post sedation and paralysis and prevent a precipitous rise in CO2 and fall in pH. They would likely also benefit from more pressure support than used in the trial (e.g. 10cm H20 rather than 5cm) to improve ventilation. Such patients may get little preoxygenation benefit from the NIV so in theory they may do just as well by simply having ventilations via BVM during the apnoeic period, except for the risk of over-ventilation and resultant aspiration caused by a human pumped-up on adrenaline. So the possible benefits of NIV in Group 3 are the potential safety benefit of machine-controlled breaths and accurately timed supported breaths, rather than oxygenation.
Group 4 – it seems rational to consider absolutely maximising your preoxygenation in patients where you expect you may end up in a bind after sedation and paralytics.
Outside of these 4 groups (especially outside of groups 1 and 2) it is unclear whether the added complexity and risks of NIV are justifiable given there may not be any substantial benefit, especially if one uses nasal prongs routinely run at ≥ 15L/min + a PEEP valve attached whenever a BVM is used and NRM run at wall flush rate.
This was an excellent study that helps us answer an important question. However its pragmatic design leaves us with one big question:
Would non-obese ED patients, not requiring oxygen, benefit from NIV compared to optimal oxygen mask therapy?
Any future ED trial would need to specifically target this group and optimise the therapies delivered to their control arm to answer this. It should also be noted that the harms in the real world from NIV are likely to be higher both due to the large number of exclusion criteria and because safety is usually diminished outside of carefully controlled trials. It is unclear whether such harm in “non-obese ED patients on room air”, would be outweighed by any actual patient outcome benefit (not just desaturations, a proxy benefit). It is also worth remembering the history of EBM tells us that all trials contain bias affecting the results that in most cases bias towards exacerbating the benefits and minimising the harms of the studied treatment (see EBM 2.0 for more information). So when mentally adjusting for this unavoidable bias, this gives further pause when considering NIV in this lowest risk sub-group.
By contrast, arguably NIV preoxygenation, should now at least be considered routinely in groups 1 and 2 and probably applied in the absence of any serious precaution or contraindication to NIV or practical obstacle to its application, as these groups have both the best prior probability for benefit and evidentiary support from this trial. This is clearly where the strongest case for practice change has been made. Where NIV is not applied, at a minimum apply optimal oxygen mask therapy discussed (non-rebreather at flush rate/apnoeic oxygenation via nasal prongs/ BVM with PEEP attached).
How might practice change actually play out?
Obesity was defined as only BMI>30. From a local perspective, about 1/3 of Australian adults are obese with a BMI>30, and this group is likely overrepresented in ED presentations and even more so in ED intubations. Consequently the combination of groups 1 and 2 (or groups 1-4) could feasibly represent say 1/3 to 1/2 of our ED intubations. If we do change practice in these groups and your department becomes very familiar with NIV pre-oxygenation, proponents may argue that it may simply be practical to standardise our peri-intubation practice and use it in all patients without contraindications, especially as optimal mask therapy (discussed above) may not be being applied consistently in the real world (as per this study’s control arm). I.e. we may end up looking for reasons not to use NIV for pre-oxygenation rather than reasons to use it.
Opposing this view, critics would cite a valid concern about the risk of underestimating real world NIV harms and practical difficulties (as explained above) for likely minimal bias-adjusted, real patient-centred benefit (as opposed to the measured proxy desaturation outcome) in these lower risk groups. Consequently, while awaiting further evidence, may choose to strictly restrict its use to groups 1 and 2 (+/- groups 3 and 4).
While we can reasonably disagree about exactly who we should apply this to, I ultimately agree we should be using NIV manifestly more frequently as preoxygenation for emergency intubation based on prior probability combined with the results of this trial.
Justin Morgenstern from First10EM has also reviewed this paper with similar enthusiasm for the findings with perhaps greater reservations about its generalisability to all ED patients.
Scott Weingart at Emcrit released a podcast on the paper including an interview with 2 authors and by contrast has a more favourable view regarding the generalisability of the findings to essentially all patients. In a separate followup podcast “wee” (within the free section), he also mentions, for those not wanting to use NIV in a particular patient, a suggested option to maximise the delivered CPAP during preoxygenation – during the final minute of preoxygenation, switch from the standard non-rebreather mask to a BVM with attached dialled up PEEP valve, manually held securely to the patient’s face (I’d add with jaw thrust), while nasal cannula are in situ underneath at 10-15L/min. This may achieve (hopefully) much of the benefit of NIV.
The above reviews are definitely worth digesting!
I have been vocal about the need for reform in Evidence Based Medicine – see EBM 2.0. There are elements of this publication that fail what I consider best practice EBM 2.0 or even best practice EBM 1.0.
- Lack of clarity
- The term hypoxaemic respiratory failure was critical to this study and its interpretation, yet was not defined anywhere in the paper.
- Post-hoc changes to the study
- In the published study protocol it states they would record “indication for intubation” in the data sheets and specifically that “hypoxaemic respiratory failure” would be recorded as an indication. I was perplexed to find then that this information was not provided anywhere in the paper or the supplementary appendix. Correspondence with Dr Gibbs revealed that, based on feedback from reviewers, this was changed post-hoc to merely to the “presence” of hypoxaemic respiratory failure because this was thought to be more objective then the subjective “indication for”. While a justifiable change, in the interests of full transparency and openness in the EBM, this should only be done if it is clearly explained in the paper, why this post-hoc change happened, and then report all the data and analysis for both “pre-change” and “post-change” outcomes. While I don’t believe in this particular study, there was nefarious intent behind this change, can you imagine if this was a drug company funded trial of a new expensive drug? To protect our patients, in such a case we must be supremely sceptical and assume such disguised non-transparent changes to the study protocol post-hoc represent misleading and deceptive behaviour to manufacture benefits that don’t exist. The EBM community must aspire to do better. For example, if in this case it was the reviewer’s direction to make this post-hoc change, the editor should have insisted on providing full transparency as described above.
